The Certificate of Analysis (COA) is a document issued by a manufacturer or a third-party testing organization that confirms a product meets its product specifications. It provides a quantified assessment of the physical and chemical properties of a product, as well as the quantities of specific active ingredients it contains.
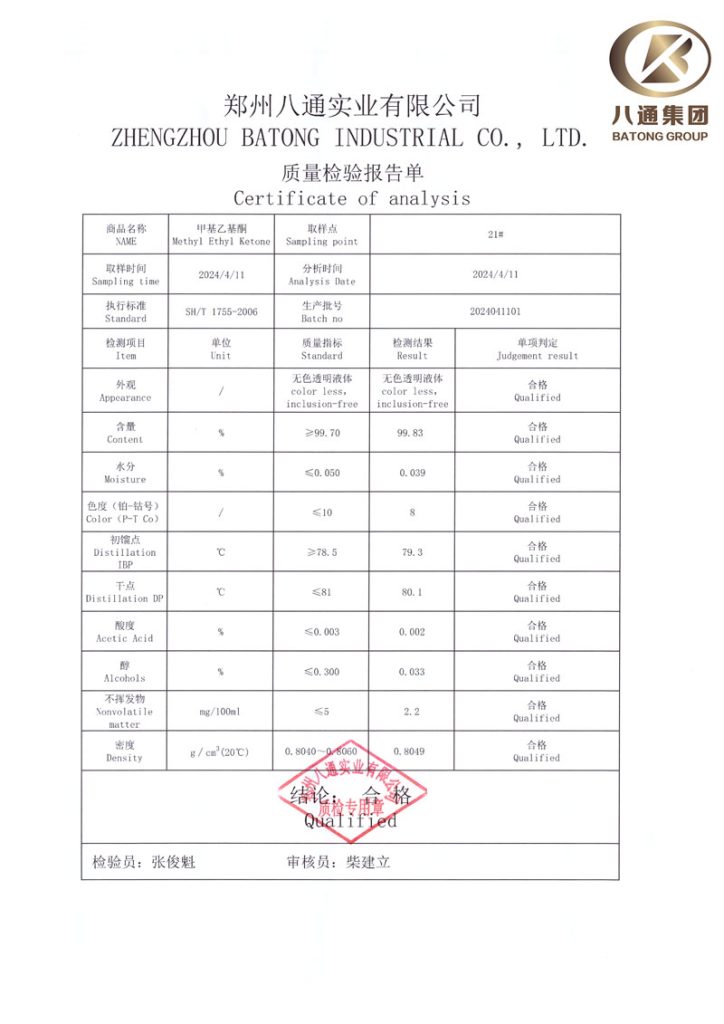
For chemical products, a COA might include information such as:
- 1. Product Information: The product's name, manufacturing date, batch number, and shelf-life.
- 2. Test Results: The results of tests conducted to evaluate the product's characteristics. This could include tests for purity, concentration, and the presence of specific ingredients or contaminants.
- 3. Methodology: The methods or standards used to conduct the tests, which might be international, national, or industry-specific standards.
- 4. Quality Assurance: Approval or certification from the individual or organization responsible for testing, often including the signature of a company representative or a lab technician.
The purpose of a COA is to provide transparency and assurance to customers that the product they are purchasing is safe, of good quality, and compliant with regulations and standards. In various industries, especially in pharmaceuticals, food and beverage, cosmetics, and chemicals, COAs are often required by regulatory bodies.












