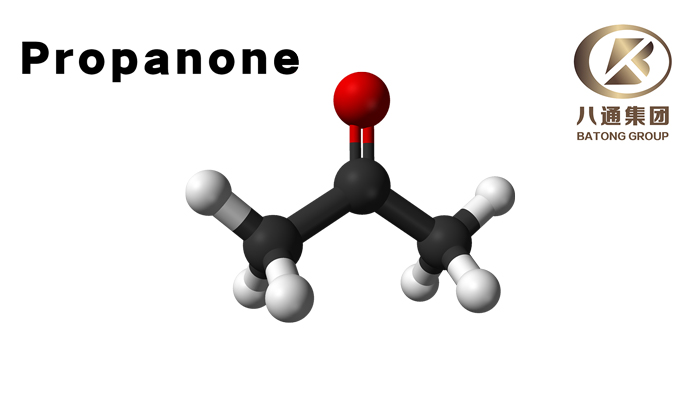
Propanone, also commonly known as Acetone, is a significant compound in the realm of organic chemistry. It is the simplest and most important of the aliphatic (fat-based) ketones. This article aims to provide detailed insights into the physical characteristics, applications, and safety measures associated with Propanone.
Propanone (CAS Number: 67-64-1, UN Number: 1090) is a colorless, volatile liquid that has a distinctive, sweet, fruity smell. It has a molecular formula of (CH3)2CO and a molecular weight of 58.08 g/mol. This flammable liquid has a melting point of -94.7 ℃ and a boiling point of 56 ℃.
One of the most notable physical properties of Propanone is its solubility. Propanone is miscible with water and serves as an excellent solvent for many organic compounds. It is also miscible with most common solvents including water, ethanol, ether, and chloroform.
Propanone's primary use is as a solvent in the manufacturing of plastics and other industrial products. It is also used in the production of pharmaceuticals, and the preparation of metal before painting. In the laboratory, Propanone is commonly used as a solvent in a variety of chemical reactions.
In conclusion, Propanone is an integral component in various industrial applications due to its excellent solvent properties. However, proper handling and storage are crucial due to its flammability and potential health hazards. Understanding the properties and proper safety measures associated with Propanone is essential for its effective and safe use.