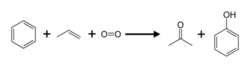
The advantage of the isopropylbenzene method is that it converts the cheaper raw materials benzene and propylene into the more valuable phenol and acetone. The other raw materials used are a small amount of catalyst, a small amount of radical-generating compounds and oxygen that can come from the air. The overall chemical reaction is summarized as follows:
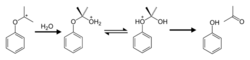
Hydrogen peroxide isopropylbenzene is hydrolyzed to phenol and acetone in the presence of an acid (e.g. sulfuric acid). The loss of the water molecule from the hydrogen peroxide evolves an electron-deficient oxygen atom. The relatively electron-rich phenyl group rearranges with this oxygen atom and evolves a more stable tertiary carbon cation; the mechanism of this step is similar to that of the Bayer-Wiliger oxidative rearrangement reaction.

The tertiary carbon positive ion is combined and rearranged with water molecules to produce an equimolar ratio of phenol to acetone.